
video 5) 5 ka table YouTube
Easy learning of Multiplication table of 1 | 1 table | one table | 1 x 1 = 1

6 ka table YouTube
0:04. 2:14. HOUSTON — Michigan 's 34-13 win against Washington served as a tidy encapsulation of the Jim Harbaugh era: flawed, a little sloppy, dotted with self-inflicted errors but ultimately.

2 se 100 tak ka table likh kar dikao Brainly.in
Out of our three weak acids, hydrofluoric acid is the strongest, so it has the largest value for Ka, but notice it has the smallest value for the pKa. The lower the value for pKa, the more acidic your acid. 3.46 is lower than 4.74, and so hydrofluoric acid is more acidic than acetic acid. Up next: video. Learn for free about math, art, computer.

2 ka table / two ka table for junior kids YouTube
The table lists the K a values and the strength of each acid and base. Strong acids are listed at the top left-hand corner of the table and have Ka values >1; Acids with a K a value less than one are considered weak and get weaker as we move to the bottom of the table.

Table of 2Table of 2 in English2 ka Pahada English mein2 ka table dikhaiye 2 ka table sunao
The Oneka Accent Table is a rectangular stool with woven cane sides. This versatile design can serve as an accent table or stool. Group in multiples to create a rectangular or larger square table. 12 Willow Lane, Nesquehoning, PA 18240. P: 800-613-3261 | F: 866-613-3264 [email protected] Products. Lighting. Furniture.

Acid Strength and Conjugate AcidBase Pairs in Chemistry
Successive acid dissociation constants are provided for polyprotic weak acids; where there is ambiguity, the specific acidic proton is identified. To find the Kb value for a conjugate weak base, recall that. Ka ×Kb = Kw (E5.1) (E5.1) K a × K b = K w. for a conjugate weak acid, HA, and its conjugate weak base, A -. Compound.
2 Ka Table Table Decorations
The larger the Ka, the stronger the acid and the higher the H + concentration at equilibrium. Like all equilibrium constants, acid-base ionization constants are actually measured in terms of the activities of H + or OH −, thus making them unitless. The values of Ka for a number of common acids are given in Table 16.4.1.

2 Ka Table Table Decorations
Table of 1 | table 1 2 | 1 ka table | Multiplication Table 1 #table | 1 time table | #multiplicationtable | 1x1 table | appendix table 1 | multiplication.

Math ka table YouTube
Howto: Solving for Ka K a. When given the pH value of a solution, solving for Ka K a requires the following steps: Set up an ICE table for the chemical reaction. Solve for the concentration of H3O+ H 3 O + using the equation for pH: [H3O+] = 10−pH (5) (5) [ H 3 O +] = 10 − p H.

2 Ka Table Table Decorations
1. Strong acids are listed at the top left hand corner of the table and have Ka values >1 2. Acid with values less than one are considered weak. 3. The strong bases are listed at the bottom right of the table and get weaker as we move to the top of the table.

9 ka table YouTube
HSO 3-. 6.3 x 10 -8. 7.2. uric. HC 5 H 3 N 4 O 3. 1.3 x 10 -4. 3.9. Ka is the equilibrium constant for the dissociation reaction of a weak acid. Here is a useful table of common Ka values of weak acids and their formulas.
katable npm
Ka Table. 1.5×10-10. Urea hydrogen ion NHCONH6.7×10-1. Zinc 2+ ion Zn2+2.5×10-10.

1 ka Table 1 ka Pahada math table 1 का पहाड़ा 1 का टेबल Todaysera
Example \(\PageIndex{1}\): Acidic Groups. Using the pK a table, estimate pK a values for the most acidic group on the compounds below, and draw the structure of the conjugate base that results when this group donates a proton. Use the pKa table above and/or from the Reference Tables.. Answer. a. The most acidic group is the protonated amine, pKa ~ 5-9

100 ka Table YouTube
This one's 10 and this one's 16. So that's a difference of six. But really each unit is in order of magnitude, so this proton on phenol is 10 to the sixth times more acidic, so this compound is one million times more acidic than ethanol. So that's really how to think about acid strength when you're looking at a pKa table.

2 ka table 10 tak 2 ka pahada dastak 2katable10tak 2kapahadadastak🙂 YouTube
pKa is an acid dissociation constant used to describe the acidity of a particular molecule. Its value is directly related to the structure of the given compound. The constant changes depending on the solvent the compound is used in. Typically, organic chemists compare the various values from their determination in water, DMSO and the gas phase.
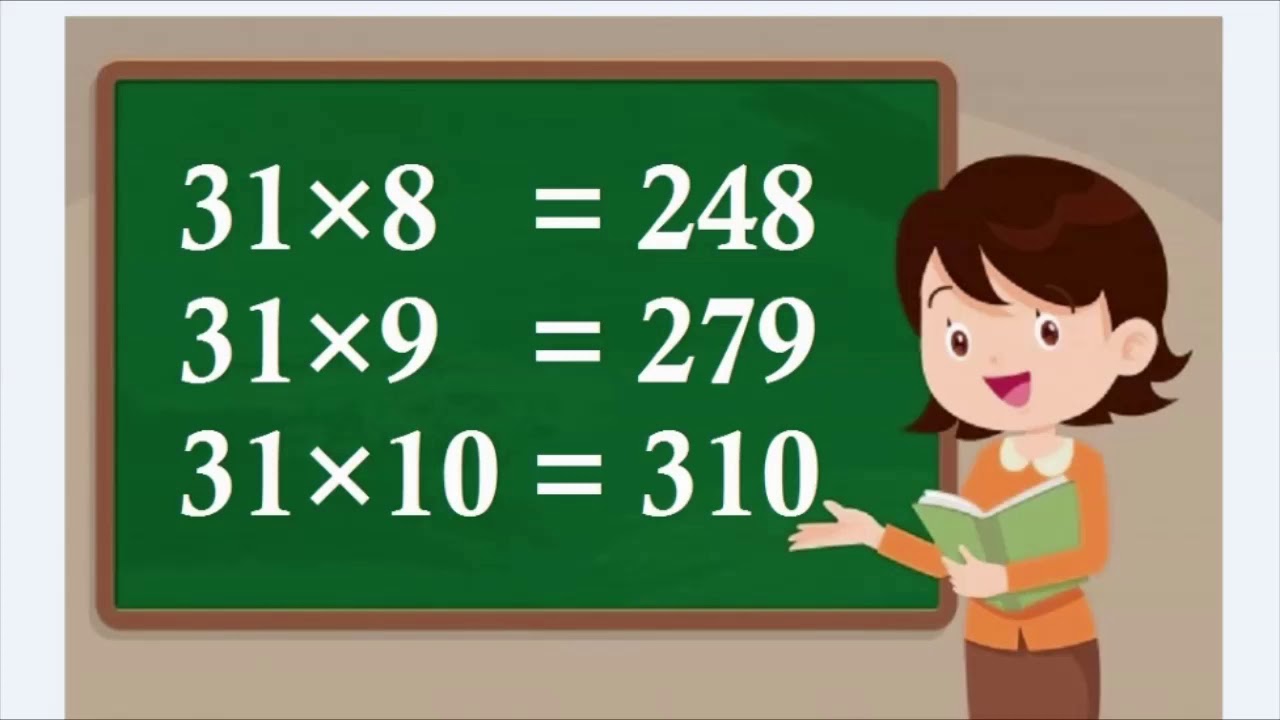
Table of 31, table of thirty one, thirty one ka table, 31 ka table YouTube
The pka of water and H 3O+ have been experimentally determined to be 14.0 and 0.0, respectively. Earlier values of 15.7 and -1.74, respectively are erroneous numbers proposed by scientists who made some errors in the calculated "rational" values.